Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


Shandong Sino Pharmaceutical Technology Co., Ltd.
We are professional supplier of Skin whitening products,Skin Rejuvenation products,Weight Less products,Peptide,Antibiotic,Analgesic,Anti-inflammatory,Vitamin,Health care products.
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


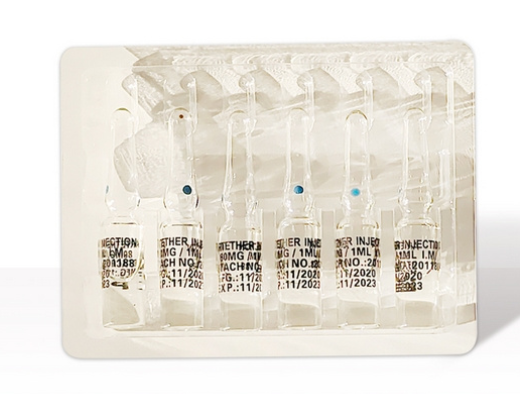
Temperature: Artemether Injection should be stored at 2-8°C (refrigerated) to maintain stability and efficacy26.
Light Sensitivity: Protect from light by keeping the product in its original packaging or using light-resistant containers16.
Sealing: Ensure the container is tightly sealed to prevent exposure to air and moisture, which may degrade the compound23.
Temperature Control: During transport, maintain a cold chain (2-8°C) using insulated containers with ice packs or refrigerated vehicles9.
Avoid Freezing: Do not freeze, as freezing may damage the product's integrity1.
Protection from Shock & Vibration: Use secure packaging to prevent physical damage during transit, especially for glass vials35.
Documentation & Labeling: Clearly label packages with "Refrigerate: 2-8°C" and "Protect from Light" to ensure proper handling5.
If exposed to higher temperatures (e.g., up to 25°C) for short periods (e.g., during transit), monitor for any signs of degradation (e.g., discoloration, precipitation)1.
Once opened or diluted (if applicable), follow manufacturer guidelines for immediate use or short-term storage.
Follow Good Distribution Practices (GDP) for pharmaceuticals to ensure quality control during shipping
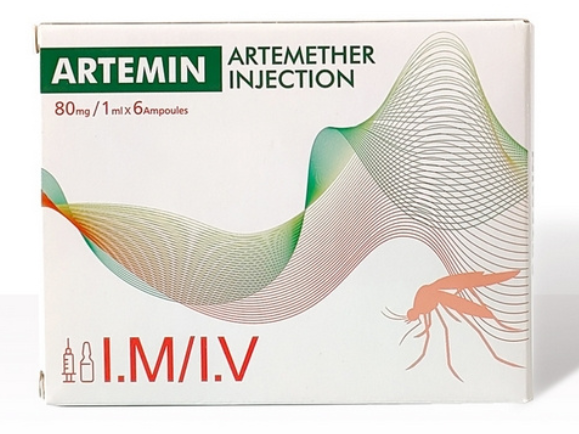
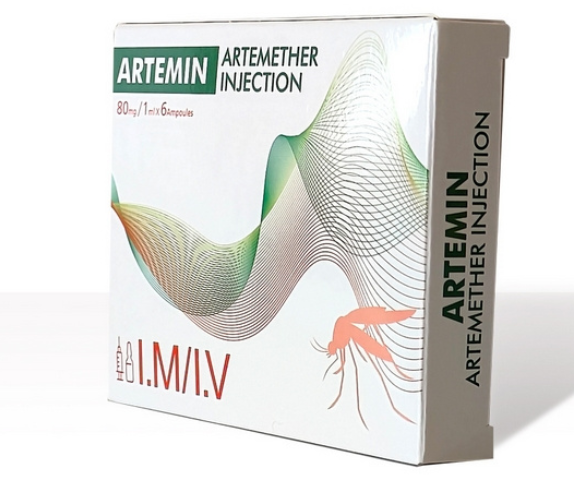
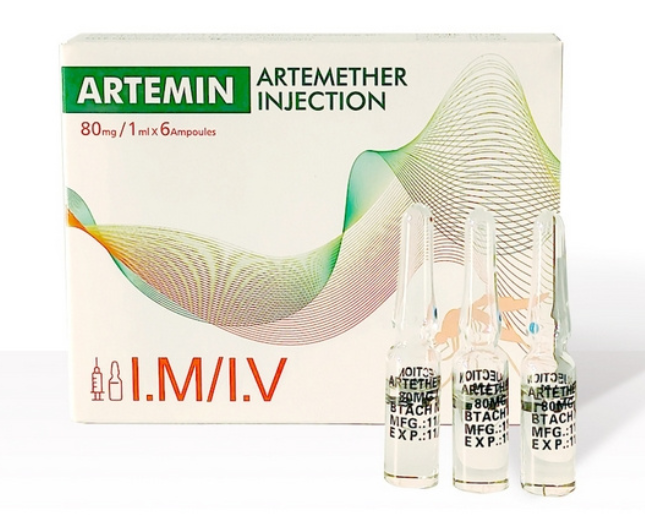
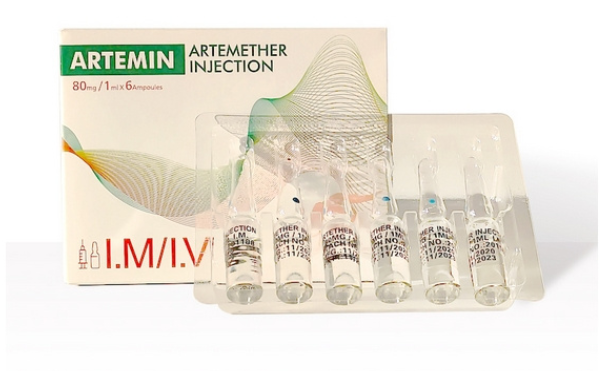

Currently, we are sourcing the distributors or agents all around the world.
Please do not hesitate to contact us immediately if you are interested in this product.
Looking forward to strong cooperation with you in the near future!
Brief Introduction to Our Company:
Shandong Sino Pharmaceutical Technology Co., Ltd. (S.S.P.T.) is a different kind of pharmaceuticals (both finished medicine products and raw materials), health products, cosmetics and medical supplies company for human needs, focusing on global markets, especially the developing world. Working with the highest levels of government and local distributors alike, we aim to not only expand our business scale and scope but also have a special interest in serving the needs of the end consumer that requires our products the most. To this end, we have a presence in 5 continents with an expanding product range to meet the demand of our main markets.
Moreover, our manufacturing facilities follow strict GMP rules and regulations. We have intergraded all GMP guidelines into internal procedures improving the degree to which results are consistently exceeding expectations. As one of the best pharmaceutical companies in China, we take our ethical obligation to improving access to high quality and affordable WHO essential drugs seriously. This is the ethical basis our company was founded on.