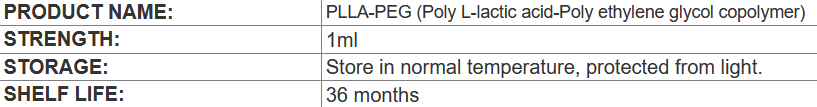

1. What is PLLA-PEG (Poly L-lactic acid-Poly ethylene glycol copolymer)
PLLA-PEG is the abbreviation of Poly (L-lactic acid)-Poly (ethylene glycol) copolymer. The microsphere mixture is a dispersion system in which the copolymer is prepared into micron-sized (typically 1-100 μm) spherical particles through a specific process (common dispersion media are water or biocompatible solvents).
Core ingredients:
1.PLLA (poly-L-lactic acid) : A biodegradable polyester material, derived from lactic acid (a natural organic acid that can be obtained through fermentation of corn, sugar beets, etc.), with lactic acid as its degradation product, and ultimately metabolized into carbon dioxide and water, having 2.no biological toxicity.
PEG (polyethylene glycol) : A hydrophilic polymer material with excellent biocompatibility, water solubility and lubricity, it is often used to improve the hydrophilicity of materials and reduce immunogenicity.
3.Copolymer structure: It is usually a "block copolymer" (such as PLLA-PEG diblock or PLLA-PEG-PLLA triblock), which combines the biodegradability and mechanical stability of PLLA with the hydrophilicity of PEG, avoiding the defects of pure PLLA such as poor hydrophilicity and too fast/too slow degradation rate
. 2.Suitable for medical and biomedical scenarios
2.Suitable for medical and biomedical scenarios1.Drug Delivery System (DDS):
Scene: Long-acting sustained-release drugs (such as anti-tumor drugs, antibiotics, hormones, and painkillers), targeted drug delivery (achieving targeting by modifying the surface of microspheres).
Advantages: Reduce the frequency of administration (such as once a month instead of daily medication), lower fluctuations in blood drug concentration, and enhance the bioavailability of drugs (avoiding first-pass effects).
PEG-PLLA microspheres loaded with paclitaxel (an anti-tumor drug) achieve slow release at the tumor site and reduce systemic toxic and side effects.
2.Tissue Engineering and Regenerative Medicine
Scene: Tissue scaffolds (such as skin, cartilage, and bone tissue repair), cell carriers (carrying stem cells and transplanting them to the damaged site, providing growth space for cells during the degradation of microspheres).
Advantages: The degradation rate matches the tissue regeneration rate, avoiding the impact of scaffold residue on tissue repair. Meanwhile, the PEG component can improve the environment for cell adhesion and proliferation.
3.Medical aesthetics field (compliant medical scenarios)
Scene: Injection filling materials (such as facial depression filling, wrinkle improvement), tissue support materials.
Advantages: Compared with traditional filling materials (such as hyaluronic acid), the degradation cycle is longer (usually 1-2 years), and the effect is more lasting. Compared with non-biodegradable materials (such as silicone), it has no long-term residue risk and is safer.
4.Biomedical testing
Scene: Antigen/antibody carriers in immunoassay, cell separation and sorting, signal amplification carriers for biosensors.
Advantages: Microspheres have a large surface area, which can load a large number of biomolecules and improve detection sensitivity. Hydrophilic surfaces reduce non-specific adsorption and enhance detection accuracy.
 3.Relevant standards and precautions
3.Relevant standards and precautionsRequirements for medical-grade materials
Raw material purity: PLLA and PEG must meet medical-grade standards (such as USP/NF, EP pharmacopoeia requirements), and there should be no excessive heavy metals or residual monomers (lactic acid, ethylene glycol).
Production standards: Production must be carried out in a GMP workshop. Key indicators such as the particle size distribution, drug loading capacity, and release rate of the microspheres must comply with drug registration standards (such as those of NMPA, FDA, and EMA).
 4.Safety of degradation products:
4.Safety of degradation products:Although the degradation product is lactic acid, high concentrations of local lactic acid may cause mild inflammatory responses. The risk needs to be reduced through formula optimization (such as adjusting the PEG ratio).
5.Storage conditions:Generally, it should be stored in a normal temperatures , avoiding light and high temperatures
Global Supplier and Exporter:
Shandong Sino Pharmaceutical Technology Co.,Ltd.
North of National Road 327, Liuhang Town, High-tech Zone, Jining, China.
 Payment: T/T or west union
Payment: T/T or west union
Delivery time: within 2 working days for ready stocks, 45 working days for batch order.
Shipment: HKEMS,TNT,UPS,door to door service for some countries.
We are looking for the agents all around the world.
Welcome to join us!
Moreover, kindly note that we are always providing our customers with Consignment Manufacturing Serivce, OEM/ODM.



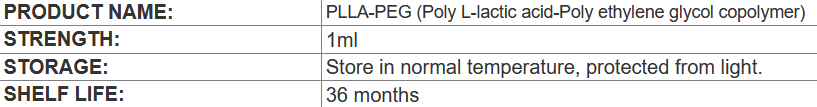

 2.Suitable for medical and biomedical scenarios
2.Suitable for medical and biomedical scenarios 3.Relevant standards and precautionsRequirements for medical-grade materials
3.Relevant standards and precautionsRequirements for medical-grade materials 4.Safety of degradation products:
4.Safety of degradation products: Payment: T/T or west union
Payment: T/T or west union