Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


Shandong Sino Pharmaceutical Technology Co., Ltd.
We are professional supplier of Skin whitening products,Skin Rejuvenation products,Weight Less products,Peptide,Antibiotic,Analgesic,Anti-inflammatory,Vitamin,Health care products.
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank



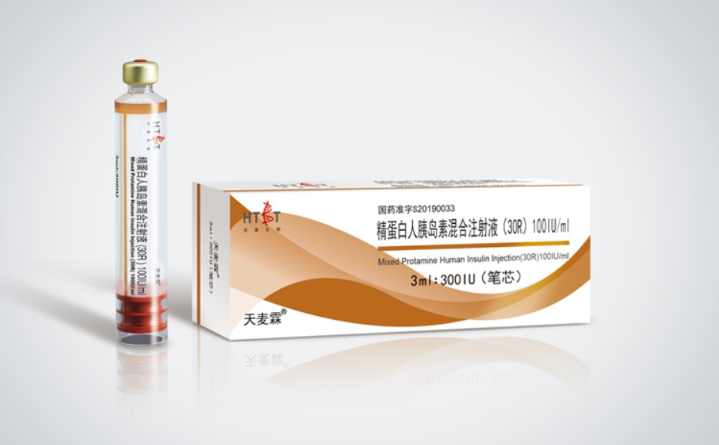
Mixed Protamine Human Insulin Injection is a medium-potency human insulin preparation. By combining human insulin with protamine (protamine) to form a suspension, it slows down the subcutaneous absorption rate and achieves basal blood glucose control within 12 to 18 hours. It is mainly used for long-term blood glucose management in type 1 and type 2 diabetes.
Core features and indications
Long-term mechanism
After insulin binds to protamine, it is subcutaneously injected to form microcrystalline precipitates, which slowly dissociate to release free insulin. There is no obvious blood drug peak (peak time is 4 to 12 hours), and the risk of hypoglycemia is lower than that of short-acting insulin, making it suitable for replacing basal insulin secretion.
Indications:
Basic blood glucose control for type 1 diabetes in adults and children;
Combined treatment for patients with type 2 diabetes when oral hypoglycemic drugs fail or there are contraindications;
Blood glucose management in special scenarios such as pregnancy and surgery associated with diabetes;
Note: Not for acute complications such as diabetic ketoacidosis (short-acting insulin intravenous infusion is required)
Contraindications and caution:
This product is contraindicated for those allergic to human insulin, protamine or excipients (those allergic to protamine should avoid using it).
2. For patients with impaired liver or kidney function, the elderly, and those during pregnancy, the dosage should be adjusted on an individual basis.
3. Contraindicated during hypoglycemic episodes.
The administration method is subcutaneous injection (abdominal/thigh/upper arm), 1 to 2 times a day (before going to bed or breakfast and dinner). Intravenous injection or intravenous drip is not allowed (the suspension is prone to clogging blood vessels).
Before use, shake well (flip 10 times + roll 10 times) to ensure the suspension is uniform (without clots or sediment), otherwise it may lead to inaccurate dosage
Dose adjustment: The initial dose should be 0.2 to 0.4U/kg body weight per day. Titrate gradually based on fasting blood glucose. When used in combination with short-acting insulin, the fasting blood glucose target should be controlled at 4.4 to 7.0mmol/L
Adverse reactions
Hypoglycemia (the most common, manifested as palpitations, sweating, dizziness, and the risk of hypoglycemia at night should be taken seriously);
Injection site reactions (redness, swelling, itching, and fat hyperplasia, requiring site rotation);
Rare protamine allergy (rash, breathing difficulties, and anaphylactic shock in severe cases
Storage and transportation specifications
Unopened: Store at 2-8 ℃ (freezing is strictly prohibited, as freezing will cause crystallization and render it ineffective).
Opened: Store at room temperature below 25℃ and use up within 4 weeks
We are supplying all of our foreign customers with Customized Pharmaceutical Formulations or Dosage Forms.
You can tell us any pharmaceutical product, no matter what it is that you want. And then we will give you a detailed solution as per your requirement.
We are always providing our customers with Consignment Manufacturing Serivce, OEM/ODM.